
Eficácia e segurança
OFEV®
Estudos Pivotais de OFEV®
FPI: A eficácia clínica de OFEV® (nintedanibe) foi avaliada em dois estudos replicados de Fase III, randomizados, duplo-cegos, controlados por placebo, com 1066 pacientes portadores de fibrose pulmonar idiopática (FPI), em 24 países (INPULSIS®-1 e INPULSIS®-2)1,2.
O estudo de fase II (TOMORROW), randomizado, duplo-cego, controlado por placebo, de determinação de dose, incluindo um grupo de dose sob OFEV® 150 mg duas vezes ao dia, fornece evidências adicionais de eficácia1.
DPI-ES: A eficácia clínica de OFEV foi estudada em pacientes com DPI-ES em um estudo de fase III randomizado, duplo-cego, controlado com placebo (estudo SENSCIS).
INPULSIS 1 E 2
Objetivo: Avaliar a eficácia e a segurança de OFEV® em pacientes com FPI.
Desenho2
Duplo-cego, controlado por placebo;
Os pacientes foram randomizados; 3:2 para receber OFEV® 150 mg duas vezes ao dia ou placebo por 52 semanas.Desfechos
Desfechos primários.2
Taxa anual de declínio da CVF.- Desfechos Secundários.2
Tempo até a primeira exacerbação aguda da FPI (reportada pelo investigador) em 52 semanas, todos os eventos foram adjudicados com análise pré-especificada de sensibilidade;
Questionário de qualidade de vida, SGRQ, do tempo inicial vs 52 semanas;
Proporção de pacientes com resposta de CVF;
Mortalidade por todas as causas relacionadas ao sistema respiratório e durante o tratamento.
INPULSIS ON: UMA EXTENSÃO ABERTA, DOS ESTUDOS INPULSIS 1 E 2
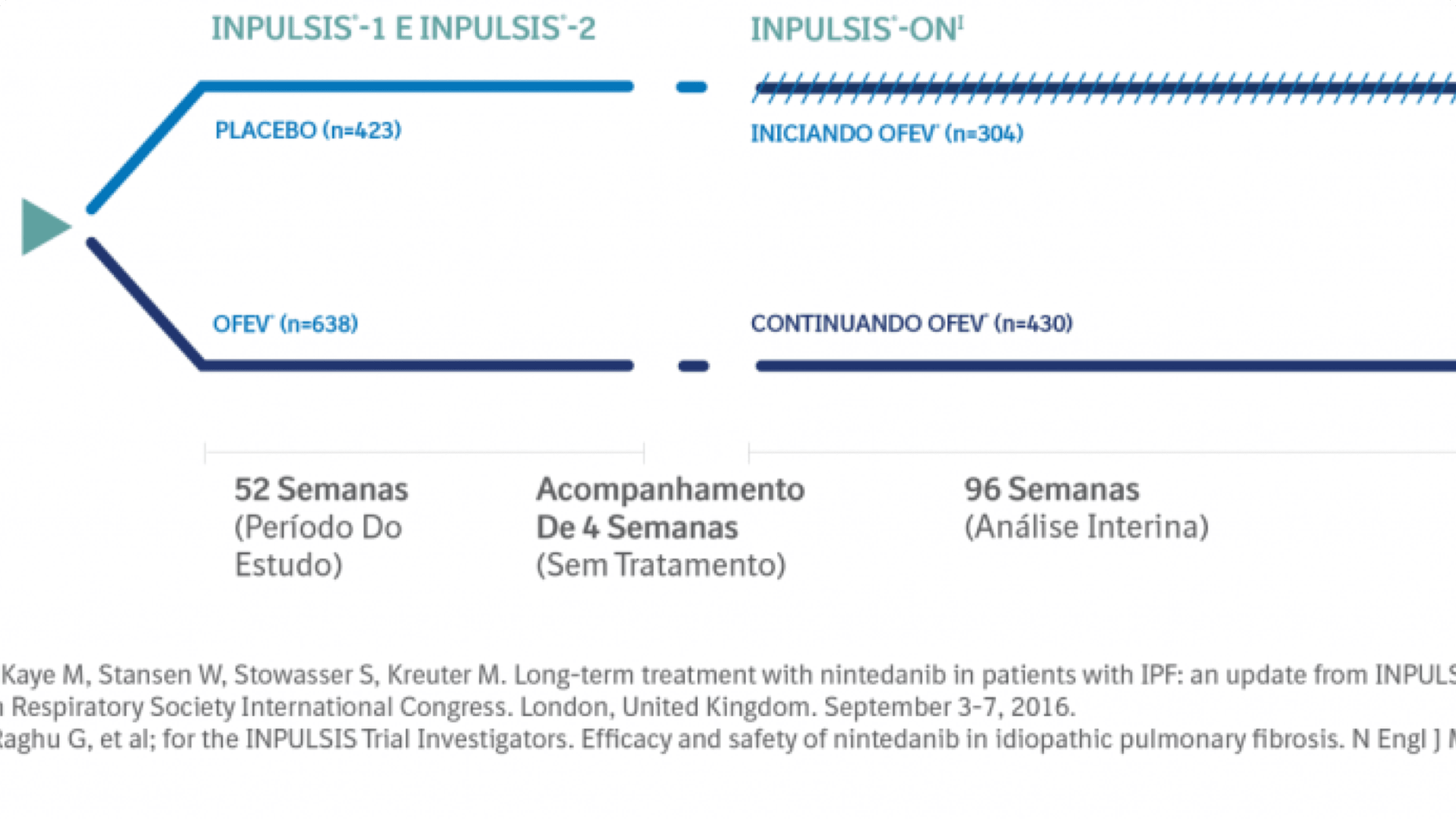
O Desfecho Primário Foi A Taxa Anual De Declínio Da Capacidade Vital Forçada (CVF).3
A taxa anual de declínio da CVF (mL/ano) foi calculada com base em um modelo de regressão linear sem imputação de valores perdidos. Todos os valores disponíveis de CFV a partir da avaliação basal até a 52ª semana foram utilizados, incluindo medidas de CVF da visita de acompanhamento para pacientes que descontinuaram a medicação do estudo prematuramente, e não completaram as visitas do estudo até a semana 52.Os Desfechos Secundários Chave Foram:3
Alteração absoluta a partir do valor basal da CVF na 52ª semana ( em mL e em % do valor predito) Proporção de pacientes com resposta de CVF, definida como aqueles com alteração na CVF (% do previsto) de ≤5% ou ≤10% Tempo até a primeira exacerbação aguda de FPI (relatado pelo investigador) durante 52 semanas Os critérios para diagnóstico de exacerbações agudas de FPI foram pré-especificados no protocolo do estudo, sendo necessária a presença de piora da dispneia no período de 1 mês, com novos achados tomográficos e tendo sido afastadas outras causas, principalmente infecção e insuficiência cardíaca. Todas as exacerbações relatadas pelos investigadores principais foram "adjudicadas" ( ou seja, revisadas) através de uma análise pré especificada por um comitê cego, afim de descartar outros diagnósticos diferenciais como Insuficiência cardíaca, infecção, etc)
Mortalidade por todas as causas e mortalidade por causas respiratórias durante as 52 semanas de tratamento Alterações a partir da avaliação basal no escore total no Questionário Respiratório de Saint George (SGRQ) em 52 semanas.
TOMORROW
Objetivo: Avaliar a eficácia e a segurança de 4 doses diferentes de OFEV® em pacientes com FPI.
Desenho1
Duplo-cego, controlado por placebo, fase II, com pacientes randomizados 1:1:1:1 para receber OFEV® 50 mg uma vez ao dia, OFEV® 50 mg duas vezes ao dia, OFEV® 100 mg duas vezes ao dia, OFEV® 150 mg duas vezes ao dia ou placebo por 52 semanas.Desfechos
Desfechos Primários.1
Taxa anual de declínio da CVF.Desfechos Secundarios.1
Tempo até a primeira exacerbação aguda da FPI (reportada pelo investigador) em 52 semanas, todos os eventos foram adjudicados como uma análise pré-especificada de sensibilidade;
Questionário de qualidade de vida, SGRQ, do tempo inicial vs 52 semanas;
Alteração desde o período basal na CVF(mL) em 52 semanas.
SENSCIS
Objetivo:
Avaliar a eficácia clínica de OFEV em pacientes com DPI-ESDesenho: 4
Os pacientes foram diagnosticados com DPI-ES com base no critério de classificação de DPI-ES de 2013 do Colégio Americano de Reumatologia / Liga Europeia Contra o Reumatismo, e com tomografia computadorizada de tórax de alta resolução ,realizada dentro dos 12 meses anteriores. Um total de 580 pacientes foram randomizados na proporção de 1:1 para tratamento com OFEV 150 mg duas vezes ao dia ou placebo duas vezes ao dia, por pelo menos 52 semanas, dos quais 576 foram tratados. A randomização foi estratificada pelo status do anticorpo anti-topoisomerase (ATA). Pacientes permaneceram cegos para o tratamento por até 100 semanas (a mediana daexposição ao OFEV foi 15,4 meses; a exposição média ao OFEV foi 14,5 meses).28Desfecho Primário: 4
O desfecho primário foi a taxa anual de declínio da Capacidade Vital Forçada (CVF) ao longo de 52 semanas. Os desfechos secundários principais foram a mudança absoluta no escore de pele modificado de Rodnan (mRSS) à partir do período basal a semana, e a mudança absoluta à partir do período basal na semana 52 o Questionário Respiratório de Saint George (QRSG). Na população geral, 75,2% dos pacientes era do sexo feminino. A média (desvio padrão [DP, min-máx]) de idade foi de 54,0 (± 12,2; 20-79) anos. Dentre todos os pacientes, 51,9% tinham esclerose sistêmica cutânea difusa e 48,1% tinham esclerose sistêmica cutânea limitada. A média (DP) de tempo desde o primeiro aparecimento de um sintoma além do fenômeno de Raynaud foi de 3,49 (1,7) anos. 49,0% dos pacientes estavam em terapia estável com micofenolato no período basal. O perfil de segurança nos pacientes com ou sem micofenolato no período basal foi comparável.28Taxa anual de declínio da Capacidade Vital Forçada (CVF)
A taxa anual de declínio da CVF (em mL) ao longo de 52 semanas foi significativamente reduzida em 41,0 mL nos pacientes tratados com OFEV em comparação aos pacientes tratados com placebo (Tabela 3), correspondendo a um efeito relativo do tratamento de 43,8%.28 O efeito de OFEV na redução da taxa anual de declínio da CVF foi equivalente nas análises de sensibilidade pré-especificadas e não foi detectada heterogeneidade nos sub-grupos pré-especificados (como idade, gênero e uso de micofenolato). Além disso, efeitos similares foram observados em outros desfechos relacionados a função pulmonar, como alteração absoluta da CVF em mL no período basal na semana 52 (Figura 1 e Tabela 4) e taxa de declínio da CVF em porcentagem do predito ao longo de 52 semanas (Tabela 5), oferecendo suporte adicional para os efeitos do OFEV em reduzir a progressão da DPI-ES. Além disso, no grupo tratado com OFEV um número menor de pacientes apresentou declínio absoluto da CVF maior que 5% do previsto (20,6% dos pacientes tratados com OFEV versus 28,5% nos pacientes tratados com placebo, razão de chancer (RC) = 0,65; p = 0,0287). O declínio relativo maior que 10% da CVF em mL foi comparável dentre os dois grupos (16,7% no grupo tratado com OFEV versus 18,1% no grupo tratado com placebo, RC = 0,91, p = 0,6842). Nessas análises, foi atribuído o pior valor do paciente durante o tratamento para os valores faltantes de CVF na semana 52. Uma análise exploratória dos dados até 100 semanas (maior duração de tratamento no estudo SENSCIS) sugeriu que o efeito do tratamento com OFEV na redução da progressão da DPI-ES persistiu além de 52 semanas. Alteração da Escala de Pele de Rodnan Modificada (mRSS) do período basal até a semana 52 A alteração da média absoluta ajustada do mRSS à partir do basal até a semana 52 foi comparável entre o grupo tratado com OFEV (-2,17 (IC de 95% -2,69 -1,65)) e o grupo tratado com placebo (-1,96 (IC de 95% -2,48 -1,45)). A diferença da média ajustada entre os grupos de tratamento foi -0,21 (IC de 95%, -0,94- 0,53; p = 0,5785)). Alteração da pontuação total do Questionário Respiratório de St. George (QRSG) do período basal até a semana 52. A alteração da média absoluta ajustada da pontuação total do QRSG à partir do período basal até a semana 52 foi comparável entre o grupo tratado com OFEV (0,81 (IC de 95% -0,92- 2,55)) e o grupo tratado com placebo (-0,88 (IC de 95% -2,58- 0,82)). A diferença das médias ajustadas entre os grupos de tratamento foi 1,69 (IC de 95% -0,73- 4,12; p = 0,1711).Análise de sobrevida
A mortalidade durante todo o estudo foi comparável entre o grupo tratado com OFEV (n= 10; 3,5%) e o grupo tratado com placebo (n = 9; 3,1%). A análise do tempo até o óbito durante todo o estudo resultou em um RR de 1,16 (IC de 95% 0,47 2,84; p = 0,7535).
INBUILD
Objetivo:
Avaliar a eficácia clínica de OFEV em pacientes com DPI fibrosantes crônicas com fenótipo progressivo.5Desenho:
Pacientes com diagnóstico clínico de DPIs fibrosantes crônicas foram selecionados se tivessem fibrose relevante (>10% de características fibróticas) na tomografia computadorizada de alta resolução (TCAR) e apresentassem critérios de progressão. Foram considerados critérios de progressão pelo menos um dos seguintes, nos últimos 24 meses, apesar de tratamento prévio: declínio relativo ≥10% do predito da capacidade vital forçada (CVF); ou declínio relativo da CVF ≥ 5 a < 10% com agravamento dos sintomas; ou declínio relativo da CVF ≥ 5 a < 10% com aumento na extensão das alterações fibróticas na imagem de tórax; ou agravamento dos sintomas respiratórios e aumento da extensão das alterações fibróticas na imagem de tórax. Pacientes com fibrose pulmonar idiopática (FPI) foram excluídos. Um total de 663 pacientes foi randomizado na razão de 1:1 para receber OFEV 150 mg duas vezes ao dia ou placebo por pelo menos 52 semanas. A randomização foi estratificada com base no padrão fibrótico na TCAR, avaliada por revisão central. Foram randomizados 412 pacientes com TCAR com padrão fibrótico semelhante a pneumonia intersticial usual (PIU) e 251 pacientes com outros padrões fibróticos na TCAR. Duas populações co-primárias foram definidas para análise neste estudo: todos os pacientes (população geral) e pacientes com padrão fibrótico semelhante a PIU na TCAR. Pacientes com outros padrões fibróticos na TCAR representaram a população “complementar”.5
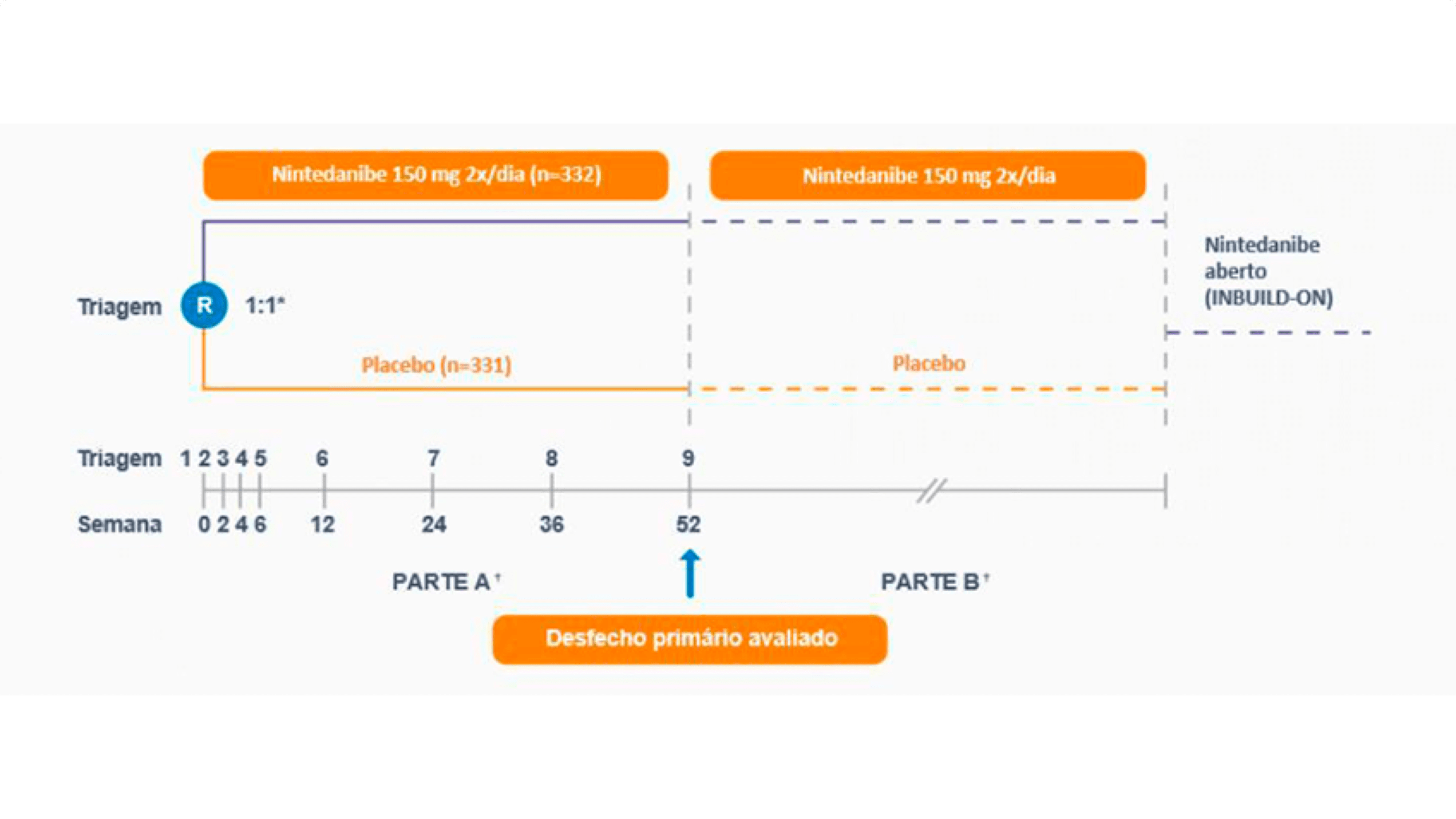
O Estudo consistiu em duas partes: a Parte A durou 52 semanas, quando o desfecho primário foi avaliado; a Parte B foi um período de tratamento variável; os pacientes permaneceram em tratamento cego durante as duas partes até que o último paciente alcançasse 52 semanas (mediana daexposição a OFEV durante todo o estudo foi de 17,4 meses e a média daexposição a OFEV durante todo o estudo foi de 15,6 meses).
Desfechos:6
O desfecho primário foi a taxa anual de declínio da Capacidade Vital Forçada (CVF), em mL, ao longo de 52 semanas.
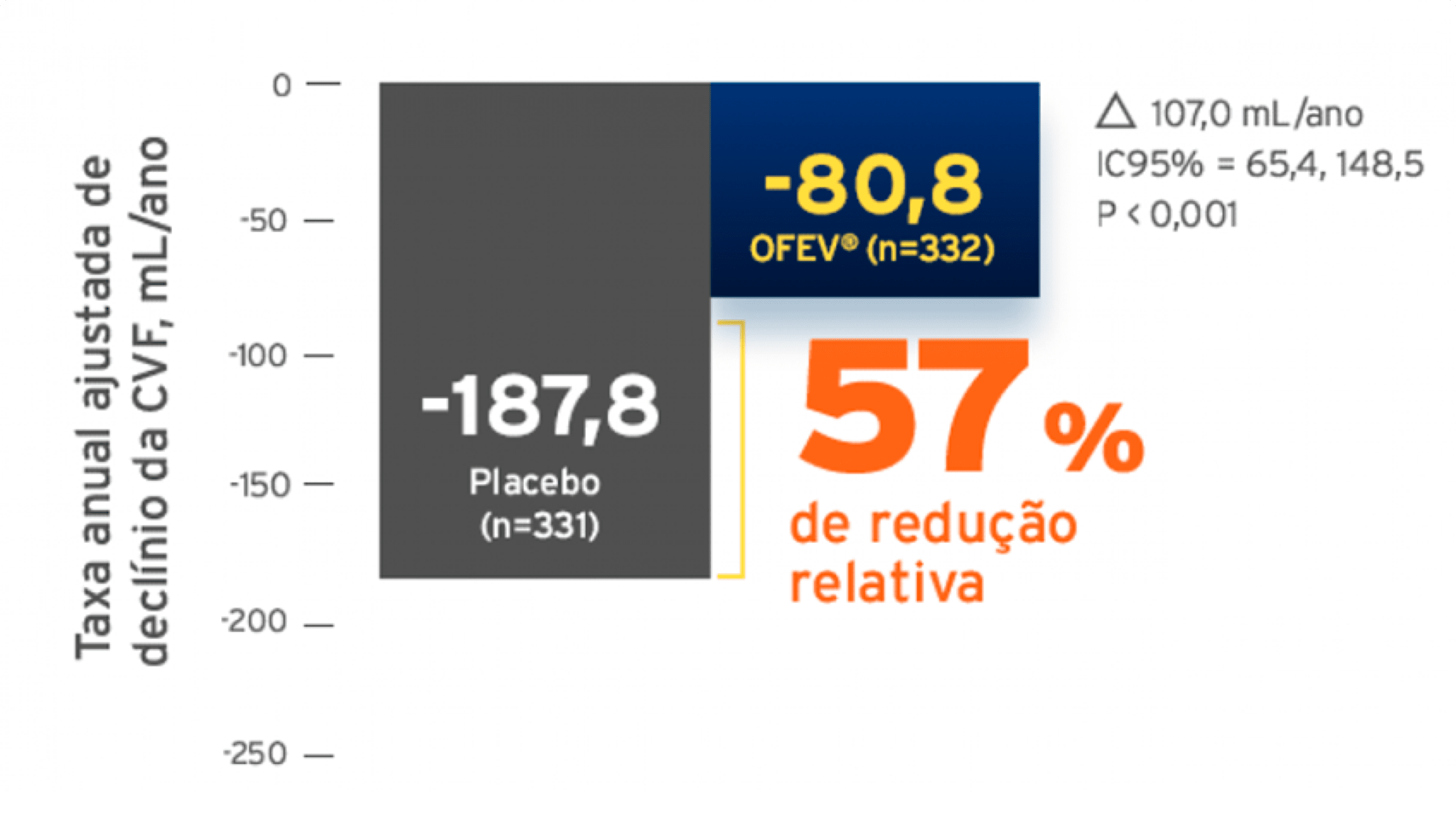
Figura 1-Taxa anual de declínio da CVF ao longo de 52 semanas: população geral. OFEV® demonstrou efeito consistente independente do padrão fibrótico da TCAR.6
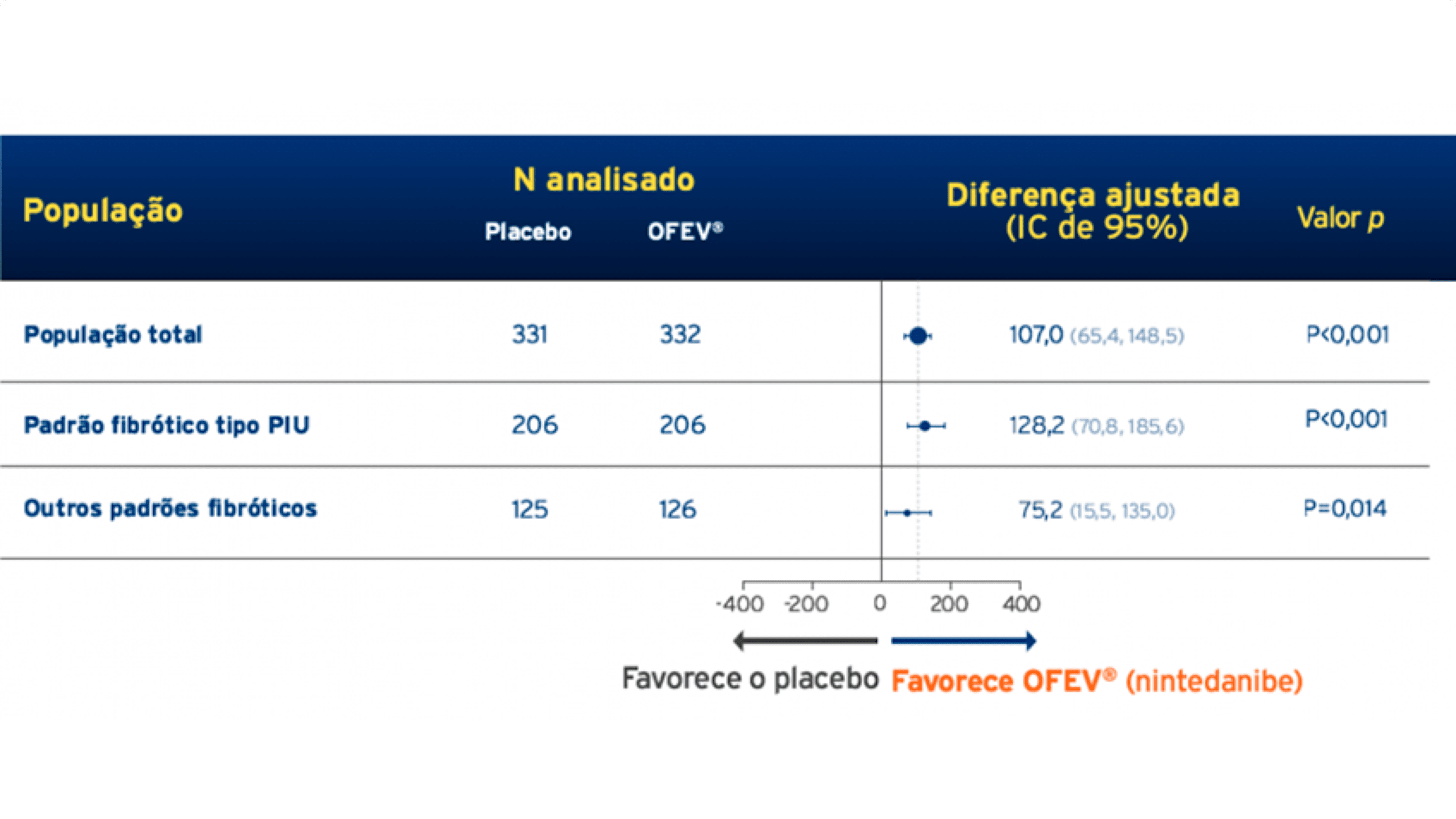
Figura 2-Taxa anual de declínio da CVF (mL/ano) ao longo de 52 semanas pelo padrão de TCAR.6
OFEV® reduziu o declínio da CVF, independente da doença de base.7
Taxa anual de declínio na CVF (ml/ano) em 5 grupos pelo diagnóstico de DPI na população geral
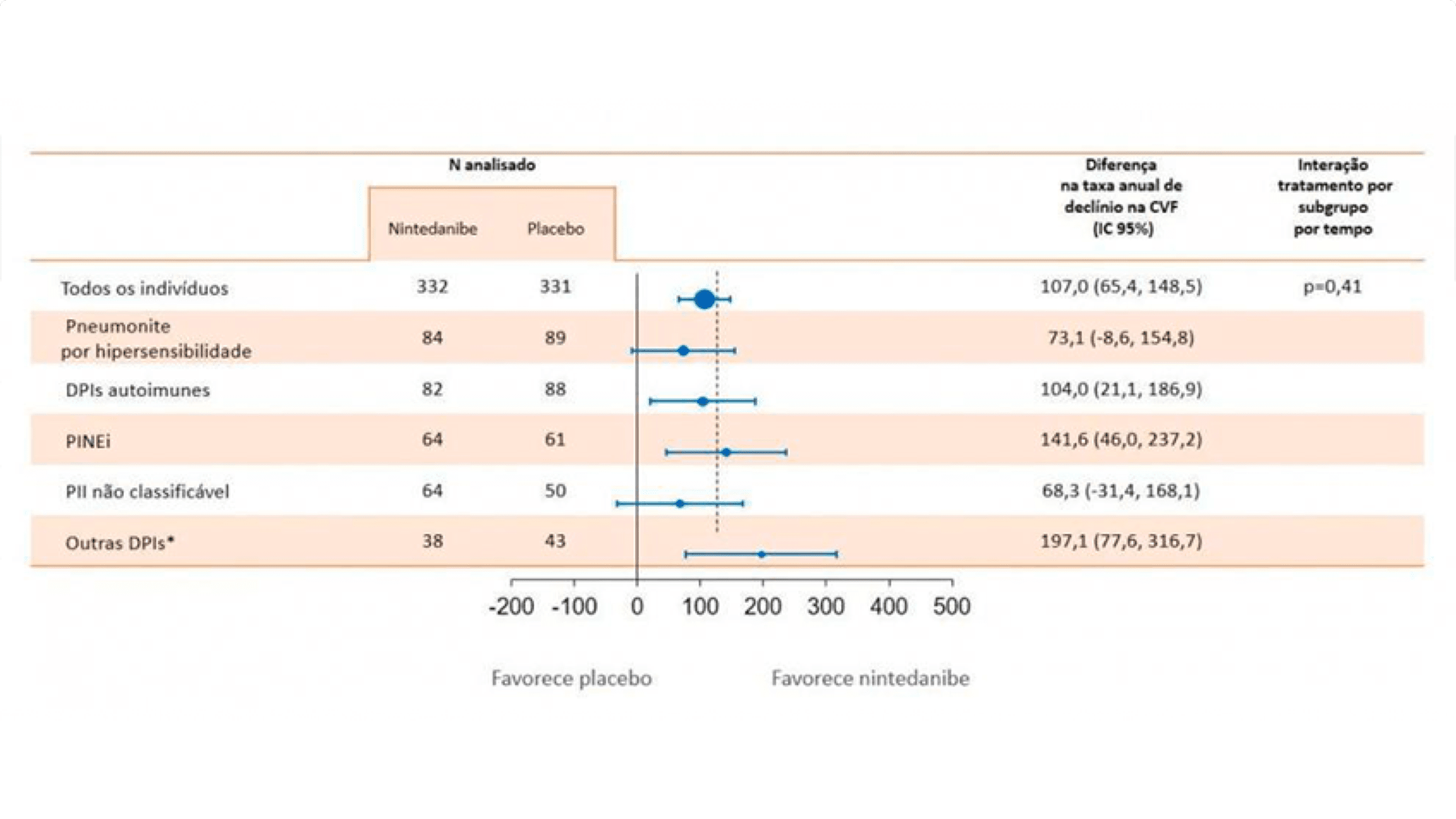
*outras DPIs: sarcoidose, DPIs associadas a exposição, entre outras.
Exacerbações agudas ou morte
Pacientes que receberam OFEV apresentaram redução de 33% no risco de exacerbações agudas ou morte.8,9Tempo para a primeira exacerbação aguda de DPI ou morte ao longo de todo estudo (partes A e B).7,8
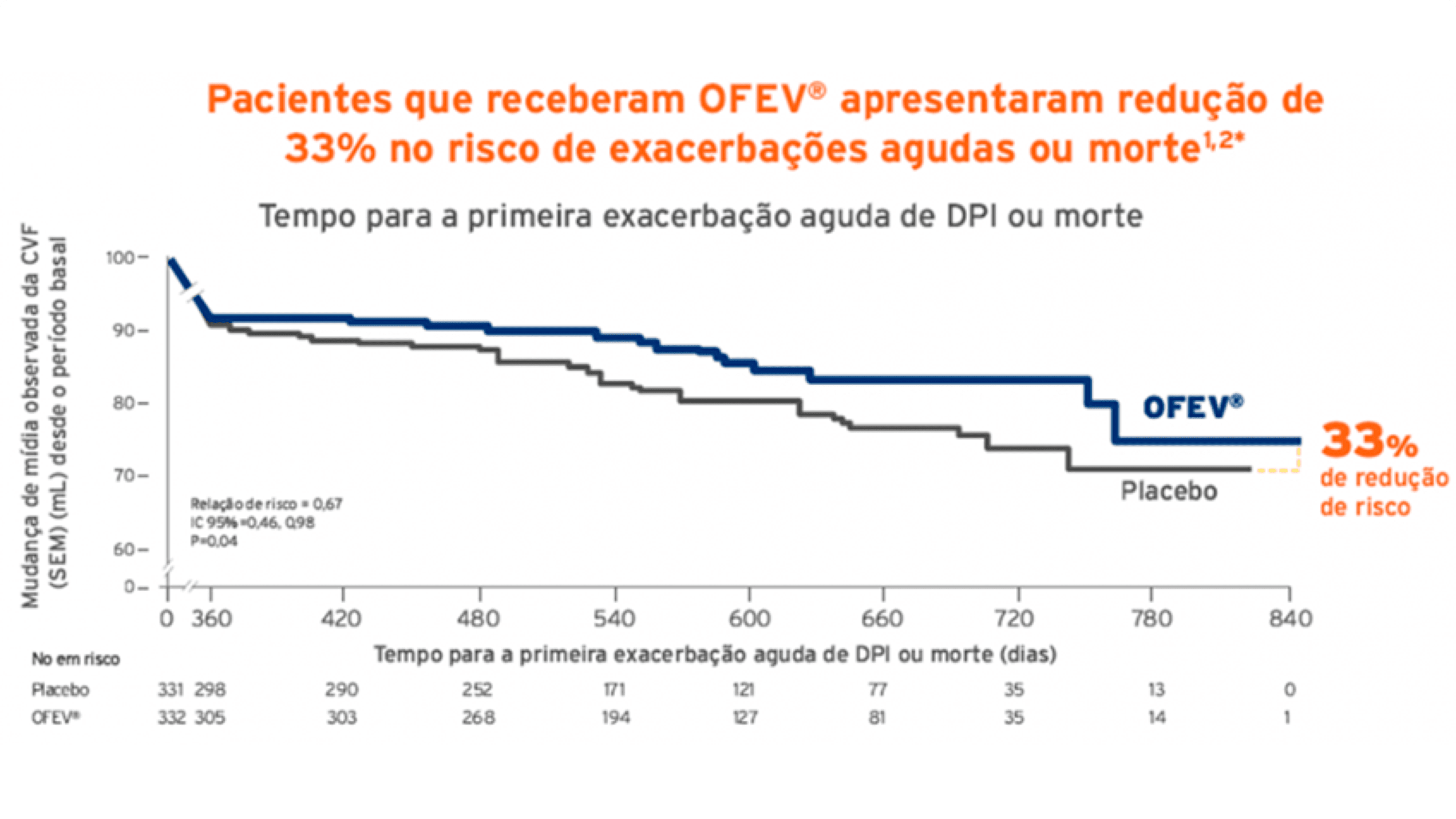
Exacerbações agudas ou morte
Pacientes que receberam OFEV apresentaram redução de 33% no risco de exacerbações agudas ou morte.8,9
Referências:
-
1.
Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079-87.
-
2.
Richeldi L, Du bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-82.
-
3.
Crestani, B, Huggins, JT, Kaye, M. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med 2019:7:60-68.
-
4.
Distler O, Highland KB, Gahlemann M, et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med. 2019; Jun 27;380(26):2518-2528. doi: 10.1056/NEJMoa1903076. Epub 2019 May 20.
-
5.
Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381:1718-27.
-
6.
Brown KK et al. Does HRCT pattern influence the effect of nintedanib in patients with progressive fibrosing interstitial lung diseases (ILDs)? Poster developed for the American Thoracic Society International Conference, 2020.
-
7.
Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallelgroup trial. Lancet Respir Med. 2020; doi: 10.1016/S2213-2600(20)30036-9.
-
8.
Bula profissional de Ofev® (esilato de nintedanibe). Versão aprovada pela ANVISA em 22Jun2020.
-
9.
Flaherty KR, Wells AU, Cottin V, et al. European Respiratory Journal Sep 2019, 54 (suppl 63) RCT1881; DOI: 10.1183/13993003.congress-2019.RCT1881
